Monthly Archives: March 2014
A significant percentage of women find that hormone therapy using birth control pills or natural progesterone supplementation is extremely effective for reducing acne blemishes and improving the overall health of the skin. It also helps to lighten periods, reduce cramping and contributes to an overall sense of well being. However, a certain subset of women experience a worsening of symptoms, increased blood pressure or feel just plain “crazy” soon after starting this type of hormone therapy. Fret not dear souls! This scenario has a simple explanation, is seemingly easy to fix and points to a situation that could build into more significant health problems later in life Progesterone, the natural form of the synthetic hormones used in birth control pills, is part of large family of hormones collectively called steroids. Most of us have heard of the more common steroid hormones like estrogen and testosterone. Others include the stress hormone cortisol and the blood pressure hormone aldosterone. When the body has an increased need for a particular hormone (for example cortisol) it can convert other hormones, like progesterone, into whatever hormone it thinks it needs. It does this with the help of enzymes. If you look at the diagram below, each arrow along the pathway is labeled with the name of the enzyme that drives the reaction. (Thanks to Walter F. Boron and Emile L. Boulpaep for their contribution of this beautiful diagram to Wikipedia. )
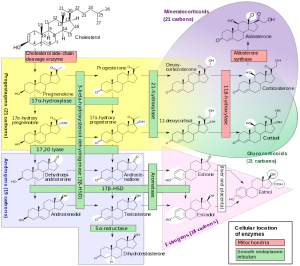 Notice progesterone, in the yellow, can convert easily to cortisol and aldosterone through the enzymes 21-hydroxylase and 11-betahydroxylase. The levels of these enzymes ultimately control how much progesterone is converted into cortisol and/or aldosterone. Sometimes there is an authentic need for more of a certain hormone. For example, higher amounts of cortisol are used by the body to moderate inflammation during an immune response or during times of increased stress. Sometimes, our genetic programming simply tells our body to make too many of these enzymes regardless of whether there is a need or not. Regardless of the cause, I am absolutely convinced that rooibos tea is an easy way to begin to slow down the conversion and depletion of progesterone. I got this idea after reading this study[i] demonstrating that rooibos tea had the ability to slow the activity of some of these enzymes and had a normalizing effect on cortisol and aldosterone precursors.
Notice progesterone, in the yellow, can convert easily to cortisol and aldosterone through the enzymes 21-hydroxylase and 11-betahydroxylase. The levels of these enzymes ultimately control how much progesterone is converted into cortisol and/or aldosterone. Sometimes there is an authentic need for more of a certain hormone. For example, higher amounts of cortisol are used by the body to moderate inflammation during an immune response or during times of increased stress. Sometimes, our genetic programming simply tells our body to make too many of these enzymes regardless of whether there is a need or not. Regardless of the cause, I am absolutely convinced that rooibos tea is an easy way to begin to slow down the conversion and depletion of progesterone. I got this idea after reading this study[i] demonstrating that rooibos tea had the ability to slow the activity of some of these enzymes and had a normalizing effect on cortisol and aldosterone precursors.
I suggested this to a few of my patients who I suspected were suffering from this specific scenario. At first, I was unsure if a simple cup of rooibos tea would be strong enough to have any significant impact. I was pleasantly surprised to find all them had noticeable results within a month. Every single one of them reported feeling calmer. Several of them reported an improvement in their sex drive. One of them had her high blood pressure return to normal within three weeks. All of them had significantly easier periods with fewer cramps and clots. One of them had a normal period for the first time in years. Previously, her periods were coming every three weeks and were accompanied by heavy bleeding. She was chronically anemic and alway felt worse with progesterone. This same lady ended up needing to reduce her dose of thyroid medication, which had been the same for years. I’m guessing this is partly because of the effects of cortisol on the thyroid through the adrenal-thyroid axis and maybe partly because progesterone boosts thyroid function. My acne patients who had symptoms of this scenario all improved by at least 20% and two ladies improved by about 70% after one month. Acne is multifaceted so it would be unlikely that rooibos tea would act as a magic pill but it definitely seems to help. I was REALLY encouraged by these results. The lady who had the high blood pressure had a relapse about two months after. At first we couldn’t figure out why but then realized that she had bought some rooibos tea that contained licorice, which is known to elevate blood pressure. When she stopped this her blood pressure returned back to normal within a week. There are a couple unknowns here. To reduce overall inflammation, I have all of my patients stop drinking coffee and alcohol and reduce their sugar intake to less than 7 grams per meal. If someone is still consuming these, I’m not sure if some of these substances might override the beneficial effects of the rooibos and continue to drive the pathway in the wrong direction. I would LOVE to hear your feedback.
Constructive comments that contribute, whether positive or negative, are welcomed. All others will be ignored or referred to trolls who are smarter than you and have nothing better to do.
[i] Schloms L, Storbeck KH, Swart P, Gelderblom WC, Swart AC The influence of Aspalathus linearis (Rooibos) and dihydrochalcones on adrenal steroidogenesis: quantification of steroid intermediates and end products in H295R cells. The Journal of Steroid Biochemistry and Molecular Biology [2012, 128(3-5):128-138]
Here is a link to my presentation. SENS6 Karen Kurtak
Hello all! This is my first presentation at a major international conference. It’s very technical but there are pieces that clarify in non-biochemical terminology . Here I present an argument for why the primary diseases of aging are not “diseases” at all but, in fact, phenotypes. I also discuss how the ketogenic diet alters signaling of DNA through nuclear transcription factors to stop, and sometimes reverse, the processes that ultimately lead to the primary “diseases” of aging including diabetes, heart disease, cancer, Alzheimer’s Disease. It was a lot of information to cover in 15 minutes but it offers a rough outline of the biochemical mechanism of action of the ketogenic diet. This took me literally over 1000 hours of sorting through science articles and plugging in the pieces until it all began to make sense. Along the way I found multiple journal articles that were completely wrong that led me down frustrating rabbit holes. Grrrr! For more extensive information please see my article that will be published in Rejuvenation Research Journal. Ultimately, this is just one example of the amount of information we already possess that is independent of clinical trials. Since I was limited to 2000 words in the article, I will be discussing each of these points in more detail in the coming months.
Thanks to Bill Andrews, who in his quest to cure aging or die trying, asked me a question that I couldn’t answer. Thank you to Aubrey de Grey for your vision that has created a firm foundation of understanding of the processes that lead to disease and aging. Thank you to all the humans of the Earth who have dedicated time and money towards uncovering truth and knowledge through science. Thank you to journals who don’t limit access of knowledge by creating pay walls. Elsevier, you guys are self-serving hijackers of knowledge. Thank you Markdavis and mmkroll for your open access photos on Flickr. Thanks to Nick, Robyn, my parents, Doreen, Bob, Michelle, Jordan, Michelle, Cliff, Darcie, Paula, Randi, Sue, Beth and Lara who supported me through multiple meltdowns and temporary possession by the Demon of OCD. Thank you Rozyln, William, Bill, (Bill’s brilliant wife whose name has escaped me), Dr. Cai, and everyone else who cheered for me before or during the conference!!!
Karen
Deeper examination of the scientific literature reveals new concerns that the food additive, azodicarbonamide, not only carries potential risks but also demonstrates that it is time for an overhaul of the FDA’s policies. This article represents scores of hours of research and triple-checking all calculations. I dedicate this to “The Food Babe”, a very courageous lady who is helping to bring awareness of the chemicals that are needlessly added into what would otherwise be considered “food”. This post demonstrates that it is time to stop this madness and allow science and logic to prevail.
I recently listened to an interview on NPR presenting the varying perspectives on a recent petition initiated by Vani, The Food Babe, asking Subway to discontinue using the chemical azodicarbonamide in their bread. I was a disappointed that no one could provide more definitive, in-depth information other to regurgitate than was already available on the PubChem or the FDA’s website. Always up for a challenge in biochemistry, I thought I would do a little research about how this stuff behaved once it entered the body. Other than its ability to act as an immunosuppressant, and being a carcinogen and irritant at unrealistically high doses, I discovered that very little was known about azodicarbonamide. After doing some calculations based on the very limited data available, I was ready to surrender any concerns I had about consuming commercial bread when I came across several articles discussing how a known carcinogen, semicarbazide, was formed during dry heating of azodicarbonamide. This was demonstrated in several separate studies by heating both commercial flours[1] and plastic seals in many twist off lids[2] to temperatures between 150−200 °C (302-422 F). The average cooking temperatures for bread are 350-375 F for home baking and 400-450 F for commercial baking. The amounts detected in the lids ranged from approximately 2.0-8.5mg per Kg (a kilogram is 2.2 pounds). Yikes, 8.5 mg/Kg is a lot! It’s a good thing we don’t eat plastic seals on lids. I wonder if any of this leeches into the contents of the jar. The amounts detected in the bread were much lower, around 0.2mg per Kg. Granted this is not a huge amount. After all, the average American “only” consumes about 1.75 pounds of bread per week. But out of curiosity, I went to Toxnet to find out the safe level of consumption for humans of semicarbazide. As of March 17th 2014 it said, “No data are available in humans. Limited evidence of carcinogenicity in animals.” Don’t confuse “limited evidence” with “the evidence suggests limited carcinogenicity”. After doing a little more digging, I discovered that the reason for this statement was two fold. First, there simply aren’t many studies investigating the “toxigenicity” or “carcinogenicity” of this known carcinogen. Second, it was difficult to assign a “safe” value because certain ethnic groups, like the Japanese, are really good at eliminating semicarbazide from their cells. Caucasians on the other hand eliminate it about half as fast as the Japanese[3]. What’s really amazing is that they have information on the exact biochemical mechanism by which it causes DNA damage but they have no information on what levels an animal or human can consume before it becomes “unsafe”.
Most animal studies performed with semicarbazide use large doses between 50 and 150 mg/kg/day. These doses are big enough that the average human is likely to never have anything close to this kind of exposure. Side effects using these big doses ranged from severe birth defects, liver hemorrhages and kidney failure[4]. In grasshoppers it was shown to have a mutagenic effect on the sperm of grasshoppers[5]. OK, fair enough! It was a grasshopper. There are no studies examining this on humans.
However, another peer-reviewed study using much smaller doses demonstrated “the incidence of lung tumors rose from 21-50% in the females and from 23-30% in the males, while the incidence of blood vessel tumors increased from 5-18% in females, but not in males” when compared to untreated controls[6]. These doses ranged from 2.2-4.0 mg/day delivered in drinking water. This is still a lot when you consider that the maximum ever found in bread was about .1 mg per pound. Nonetheless, since this is a ‘known carcinogen” I thought I would see if there was any evidence of bioaccumulation. Bioaccumulation studies are performed on many toxic chemicals to make sure that they can’t accumulate over time to levels that are toxic. Hmmmm…no bioaccumulation studies for semicarbazide (or azodicarbonamide). However, we do have one study dating all the way back to 1963 showing that .3-1.4% of hydrazine (a close relative of semicarbazide which is metabolized through the same biochemical pathways) remained in a mouse carcass 48 hours after administering doses[7]. Since the research hasn’t been performed we have to make a couple of assumptions here but let’s pretend that the clearance rate of hydrazine is similar to semicarbazide.
Here is simplified version of the calculation: (It’s really boring and nerdy. If you prefer to skip the math, the answer is at the bottom of the paragraph. For other biochemists out there…yes, enzyme kinetics is slightly more complicated but this is completely within the realm of possibility)
From earlier, the average American could conceivably consume about .175 mg per week of semicarbazide by eating 1.75 pounds of bread per week that contains azodicarbonamide. We can break this down to 0.025 mg per day or 0.050mg every 48 hours. The mean average of hydrazine retention (and presumably semicarbazide retention) is .85% every 48 hours SO 0.05mg consumed every 48 hours times .85% (.0085) leaves .0015mg remaining somewhere in the body every week. In order to accumulate the lowest dose that can potentially cause health problems 2.2mg, the average American would have to eat 1.75 pounds of bread per week for 28 years and 73 days. If they were the type of person who eats bagels for breakfast, a Subway sandwich for lunch and pizza for dinner, they might accumulate these levels in under 5 years.
The average lifespan of a laboratory mouse is three years and they are kept in a sterile environment where they are not exposed to any other chemicals that might interfere with the experiment. Humans, by contrast, are messy to study. We are exposed to small amounts of thousands of chemicals everyday that could interfere with any well-intentioned lab experiment. This brings me to my next point. As I mention above, both semicarbazide and azodicarbonamde are part of a larger family of chemicals called hydrazines. This whole family of chemicals is metabolized (broken down) by enzymes in the digestive system and liver using a process called acetylation. Think of acetylation like a toilet that flushes these chemicals out of the body. As long as there is enough room in the toilet and enough water to eliminate these chemicals, then everything is hunky dory. But what happens if there are more chemicals than the toilet can effectively eliminate? Other chemicals that are also eliminated through this pathway include many weed killers, acetopminphen (aka Tylenol™), brain chemicals like seretonin and dopamine, the allergy chemical histamine and carcinogenic heterocyclic amines that are produced by cooking meat at high temperature. The body can produce more enzymes as it needs to keep up with these demands but, like all things in Nature, there is a point at which the system becomes overwhelmed. In other words, the toilet begins to back up.
Another BIG concern I have is that we study the doses of these things that kill us or give us cancer (sometimes) but we don’t study the doses in which they start to negatively affect the body. We know that semicarbazide potently inhibits the function of an enzyme called SSAO that is used to break down histamine (the allergy chemical) in vascular tissue[8]. (Hmmm, that’s a strange coincidence. The studies performed on mice produced tumors in vascular tissues.) Would small but ongoing inhibition of SSAO cause a net overall rise in histamine levels? OR would chronic exposure to semicarbazide cause chronically elevated levels of SSAO leading to damage to vascular tissues. According the theory of enzyme kinetics, maybe both. Excess activity of SSAO has been associated with diseases like neuropathy and Multiple Sclerosis[9]. If we ignore the fact that some unknown chemical might be triggering the increased activity of SSAO, we are currently looking at using semicarbazide as a drug to help these conditions[10]. Perhaps we will find that people with these diseases can benefit from increasing their consumption of azodicarbonamide-containing bread.
Using science and logic, the only possible conclusion that we can draw about the safety of adding azodicarbonamide to bread is that we have no idea what amount is safe, especially over the long term. The data is not available because the studies haven’t been performed. Granted, the studies cited on the FDA’s website are “peer-reviewed” but 95% of them were performed before 1986 on mice. The average laboratory mouse lives 1.3-3 years. This is no way is representative of a human lifespan or the daily barrage of chemical challenges posed by “living” in an age where simply breathing the air is its own science experiment. Since the 1980’s science has evolved so quickly that it has practically broken Moore’s Law. It is time that the recommendations made by the FDA are brought up to date to reflect the science of the new millennium and to include studies that more accurately reflect human lifespan and real-world scenarios. At the very least all man-made chemicals that are included in our food should have bioaccumulation data. There is no doubt that the FDA serves and has served as a vital part of human health and survival in the United States. However, it is time for the administration that has persisted in cloaking parts of itself and of the chemical industry in antiquated dogma to either step into the new millennia and perform its duties for “the people” or to step aside and allow science and logic to prevail.
Constructive comments that contribute to the advancement of knowledge are gratefully accepted.
[1] Becalski A., Lau B.P.Y., Lewis D., Seaman S.W.Semicarbazide Formation in Azodicarbonamide-Treated Flour: A Model Studylski A., Lau B.P.Y., Lewis D., Seaman S.W. J. Agric. Food Chem., 2004, 52 (18), pp 5730–5734
[2] Stadler H., Mottier P., Phillippe G., Gremaud E., Varga, N., Laljie S., Whitaker., Kintscher J., Dudler, V., Read W.A., Castle L., Semicarbazide is a minor thermal decomposition product of azodicarbonamide used in the gaskets of certain food jars Analyst, 2004,129, 276-281 DOI: 10.1039/B314206J
[3] Koizumi A1, Nomiyama T, Tsukada M, Wada Y, Omae K, Tanaka S, Miyauchi H, Imamiya S, Sakurai H. Evidence on N-acetyltransferase allele-associated metabolism of hydrazine in Japanese workers. J Occup Environ Med. 1998 Mar;40(3):217-22.
[4] Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Man. Geneva: World Health Organization, International Agency for Research on Cancer, 1972-PRESENT. (Multivolume work). Available at: http://monographs.iarc.fr/index.php p. S7 71 (1987)] **PEER REVIEWED**
[5] Bhattacharya, K. Chromosome damage induce by semicarazid in spermatocytes of a grasshopper. Mutat Res. 1976 Jul;40(3):237-42.
[6] . Toth B., Shumizu H., Erickson J. Carbamylhydrazine hydrochloride as a lung and blood vessel tumous inducer in Swiss Mice. Eur J Cancer. 1975 Jan;11(1):17-22.
[7] Thomas Dambrauskas and Herbert Cornish The Distribution, Metaboism and Excretion of Hydrazine in Rat and Mouse. Toxicology and Applied Pharmacology 6, 653-663 (1964)
[9] Wang E. Y., Gao H. , Slater-Cid L., Zhang J, Huang L., Podar E.M., Miller A., Zhao J., O’Rourke A., Linnick M. D. Design, Synthesis, and Biological Evaluation of Semicarbazide-Sensitive Amine Oxidase (SSAO) Inhibitors with Anti-inflammatory Activity J. Med. Chem., 2006, 49 (7), pp 2166–2173 DOI: 10.1021/jm050538l
[10] Kinemuchi H, Sugimoto H, Obata T, Satoh N, Ueda S. Selective inhibitors of membrane-bound semicarbazide-sensitive amine oxidase (SSAO) activity in mammalian tissues. Neurotoxicology 2004 Jan;25(1-2):325-35.