Monthly Archives: January 2015
In the temperate zones of the Earth, late summer into autumn has been a time of celebration in many cultures. This is the time when all creatures breathe a sigh of relief as the hard work of growth slows. The cooler air transforms summer’s searing rays of sunshine into loving, golden warmth. Pregnant with sugar, fruits of flowering plants hang heavy from the branches and dapple the landscape in a mosaic of reds, blues and purples from anthocyanins and carotenoids. On the ground, combinations of lutein and zeaxanthin color the winter squashes of the Cucurbita family with the same oranges and yellows that are revealed as chlorophyll relinquishes its dominion over the foliage.
Colorful pigments that once acted as a beacon for pollinators in an array of colors and hormones[1] assume a new form that will serve as this year’s bridge of survival for numerous species of birds and mammals, including humans.
Over these precious few weeks, concentrated glucose and fructose flow in like the ocean tide. With them, the stomach’s master hormones of appetite flip flop. Ghrelin’s waxing and leptin’s waning[2] impose an ever-rising voracity of appetite that has driven successful survival of species over hundreds of millions of years. Inside the sweet goodness lurks even more treasures. Fresh omega-six oils from seeds and grains give a fresh boost to dwindling eicosanoids that are crucial for cell-to-cell communication. Vitamin E, selenium[3], vitamin C and phytonutrients stand like a levy to ensure the rising tide of inflammation doesn’t breach its banks.
In Traditional Chinese Medicine Theory, this time of year was considered the fifth season associated with the Earth element. Warmth, sunshine, water and Earth have been magically transformed by a billion tiny seeds into a form that passes life’s nourishment unto us. In the Jewish tradition, this season beckons the new year known as Rosh Hashanah.
“Blessed are you, sovereign of the Universe who brings forth bread to the Earth…who has kept us in life, has sustained us and brought us to this season.” Torah
Lurking deep within the cell, all the way down to the nuclear membrane, a sugar-laden surge of insulin nudges a sleeping Goddess from her torpor. 2.1 billion years ago[4],[5] some of the earliest fungi birthed this goddess and time kindly bequeathed her unto humans. In science she is known as SREBP or sterol regulatory elemental binding proteins. She is the one who, as if by magic, signals that transformation of sugar into a form that can be stored for later use as triglycerides[6] and fat[7]. Without her, most animals in the temperate and arctic zones are unlikely to survive even one winter.
Because of SREBP’s, every cell can make its own LDL cholesterol for membrane repair and vitamin D synthesis. However, without a way to supply basic antioxidants to the cell, LDL quickly oxidizes. This transformation from Dr. Jeckel to Mr. Hide damages everything it touches[8] and is considered to be one of the driving forces of atherosclerosis7. In order to protect her inner world and ensure a constant supply of antioxidants, SREBP must ask for a little help from one of her cousins in the liver, SREBP-1. While most of the cells of the body settle for glucose as an energy source, the liver engages in a more refined taste for fructose. In fact, liver cells are the only ones that can use fructose and its effects are incendiary. Fructose drives rapid production of LDL cholesterol, fats and inflammation in the liver[9],[10]. This preference for fructose acts as a supply chain for the trillions of cells’ insatiable need for antioxidants during times like these. But without SREBP, these antioxidants are useless. She alone is the key master who permits passage of these antioxidants across the cell membrane. Under the dominion of SREBP, the LDL cholesterol receptor rises to the surface of the cell like a fish rising to feed. If it is lucky, LDL cholesterol will land in its mouth. Along for the ride, precious antioxidants like vitamins A, C, and E are granted access to the cell’s inner world[11].
As this season wanes, berries hang dried and scant on the branches. Insulin recedes as the sugar festival comes to a close. The Earth cools. SREBP breathes a deep sigh as her hard work comes to an end. As she falls into her winter nap, she brings many of the creatures of the Earth with her. Only one creature has successfully escaped the dominion of this goddess. Humans innovated to store carbohydrates externally. This consistent supply of sugar drives insulin to ensure that SREBP never sleeps. Her unrelenting state of slavery drives disorders like obesity[12],[13], fatty liver[14], insulin resistance[15] and atherosclerosis[16], [17]. Perhaps this goddess would argue that these are not diseases at all but are phenotypes brought on by depriving her of a proper rest.
[1] Cutler A.J., Krochko J.E. Formation and breakdown of ABA. Trends Plant. Sci. 1999;4:472–478. doi: 10.1016/S1360-1385(99)01497-1
[2] Teff KL, Elliott, SS, Tschop M, Kieffer TJ, Rader D., Heiman M., Townsend RR., Keim NL, D’Alesso D, Havel Dietary fructose reduces circulating insulin and leptin, attenuates postprandial suppression of ghrelin, and increases triglycerides in women. PJ J Clin Endocrinol Metab. 2004 Jun;89(6):2963-72
[3] Giacomo dugo, Lara La Pera, Donatella Pollicino, marello Saitta. Determination of Selenium Content in Different Types of Seed Oils by Cathodic Stripping Potentiometry (CSP) J. Agric. Food Chem., 2003, 51 (19), pp 5598–5601
[4] Timothy F. Osborne, Peter J. Espenshade Evolutionary Conservation and Adaptation in the Mechanism that Regulates SREBP Action: What a Long Strange tRIP It’s Been. Genes & Dev. 2009. 23: 2578-2591, doi:10.1101/gad.1854309
[5] V Laudet Evolution of the Nuclear Receptor Superfamily: Early Diversification from an Ancestral Orphan Receptor. Journal of Molecular Endocrinology Dec. 1, 1997. 19 2-7-226
- [6] Colleen K. Nye Glyceroneogenesis Is the Dominant Pathway for Triglyceride Glycerol Synthesis in Vivo in the Rat The Journal of Biological Chemistry, 283, 27565-27574. October 10, 2008
[7] Hitoshi Shimano, SREBPs: physiology and pathophysiology of the SREBP family. The FEBS Journal 2009 276:3 616-621
[8] Low Density Lipoprotein Can Cause Death of Islet β-Cells by Its Cellular Uptake and Oxidative Modification Miriam Cnop, Jean Claude Hannaert, Annick Y. Grupping, and Daniel G. Pipeleers Endocrinology 2002 143:9 , 3449-3453 http://dx.doi.org/10.1210/en.2002-220273
[9] Zhang C, Chen X, Zhu RM, Zhang Y, Tu T, Wang H., Zhao H, Zhao M, Ji YL, Chen YH, Meng XH, Wei W, Xu DX. “Endoplasmic reticulum stress is involved in hepatic SREBP-1c activation and lipid accumulation in fructose-fed mice.” 2012 Aug 3;212(3):229-40. doi: 10.1016/j.toxlet.2012.06.002. Epub 2012 Jun 12.
“ER stress contributes, at least in part, to hepatic SREBP-1c activation and lipid accumulation in fructose-evoked NAFLD.”
[10] Koo HY, Miyashita M, Cho BH, Nakamura MT. Replacing dietary glucose with fructose increases ChREBP activity and SREBP-1 protein in rat liver nucleus. 2009 Dec 11;390(2):285-9. doi: 10.1016/j.bbrc.2009.09.109. Epub 2009 Sep 30.
“Nuclear SREBP-1 was 2.2 times higher in fructose-fed rats than glucose-fed rats.”
[11] Maret G Traber, Herbert J Kayden “Vitamin E is Delivered to Cells via the High Affinity Receptor for Low-Density Lipoprotein” The American Journal of Clinical Nutrition 40: October 1984, pp 747-51.
[12] Hitoshi Shimano, SREBPs: physiology and pathophysiology of the SREBP family. The FEBS Journal 2009 276:3 616-621
[13] Hitoshi Shimano, SREBPs: physiology and pathophysiology of the SREBP family. The FEBS Journal 2009 276:3 616-621
[14] Moon YA, Liang G, Xie X, Frank-Kamenetsky M, Fitzgerald K, Koteliansky V, Brown MS, Goldstein JL, Horton JD. The Scap/SREBP pathway is essential for developing diabetic fatty liver and carbohydrate-induced hypertriglyceridemia in animals. Cell Metab. 2012 Feb 8;15(2):240-6
[15] Iichiro Shimomura, Robert E. Hammer, James A. Richardson, Shinji Ikemoto, Yuriy Bashmakov, Joseph L. Goldstein,Michael S. Brown
Insulin resistance and diabetes mellitus in transgenic mice expressing nuclear SREBP-1c in adipose tissue: model for congenital generalized lipodystrophy. Genes Dev. 1998 October 15; 12(20): 3182–3194.
[16] Karasawa T, Takahashi A, Saito R, Sekiya M, Igarashi M, Iwasaki H, Miyahara S, Koyasu S, Nakagawa Y, Ishii K, Matsuzaka T, Kobayashi K, Yahagi N, Takekoshi K, Sone H, Yatoh S, Suzuki H, Yamada N, Shimano H. Sterol regulatory element-binding protein-1 determines plasma remnant lipoproteins and accelerates atherosclerosis in low-density lipoprotein receptor-deficient mice. Arterioscler Thromb Vasc Biol. 2011 Aug;31(8):1788-95.
[17] Kurtak, K. Dietary and Nutritional Manipulation of the Nuclear Transcription Factors, PPAR’s and SREBP’s, as a Tool for Reversing the Primary Diseases of Premature Death and Aging. Rejuvenation Research 17-2. April 2014. P 140-44.
A wise shaman once said, “Don’t confuse compassion with sacrifice. The divine mother is a warrior who is fierce, fearless and full of compassion.” She does not bring forth life into this world with the dainty beauty that has befallen the stereotype of women. She births life as a bloody, ripping warrior whose battle cries reverberate through her body with the echo of a billion lives that came before her. Some made it. Most never survived that journey. Under the escort of Kali, they returned into loving arms of the place from where we all came. And here you stand on this Earth. One of the few whose DNA survived the journey over billions of years. One who survived the War of Nature[1] long before mammals even existed. Who survived famines and floods, droughts and disease, fires, volcanoes, and ice ages. One who defied predation. Much later your genetic material survived tribal wars and world wars, injustices, abuses and epidemics. Your DNA even survived childbirth. Along the journey, your triumphs picked up many friends along the way.
In 1953, Watson and Crick’s discovery of DNA opened our naïve minds to the possibility that life could be as simple as a blueprint. Seemingly endless combinations of codons have been passed from generation to generation from the genesis of life itself. As we sorted through the simplicities of blue or brown eyes, red or blond hair, our genome taught us that an entire existence has come with us that we still don’t completely understand. What we once thought was “junk DNA” was later discovered to be our viral ancestors. One was given the name ERVWE1. Her very presence birthed our own ability to carry out placental development and thus, embryo survival[2]. Through coding for a protein called syncytin[3], she grants sperm and egg another chance to come together for another shuffle of adenine, cytosine, guanine and thymine. Without her, mammalian mothers cease to exist.
Later we discovered completely different type of DNA inside each cell that has nothing to do with the color of our eyes. This DNA resides inside the cell’s mitochondria and enables almost all living beings to convert food into energy. It is a bridge for nutrients between our inner world and Mother Earth. Whether it autogenously developed on its own or came from an ancient bacterium is still a mystery. Unlike the DNA that is passed on from the union of male and female, mitochondrial DNA comes exclusively from the mothers who have passed it through ova from generation to generation.
Another universe of genetic material that is passed from the mother is the microbiome. This is “the ecological community of commensal, symbiotic, and pathogenic microorganisms that literally share our body space.”[4],[5]. These various bacteria, fungi, parasites and viruses provide signaling that influences the inner workings of our entire body. They support immune function, moderate inflammatory responses, generate vitamins that we are not capable of making, produce hormones from some of the foods we eat, help us to absorb minerals, and regulate the production of neurotransmitters. Most importantly, they allow our immune system to remain competitive with the rate of evolution of pathogens.
Comprised of the same creatures found in the Earth itself, this genetic material that we carry inside us has been passed down through thousands of generations. Most animals on the planet, including many (and possibly all) born through eggs[6] receive this life-giving inoculation of Earth as they pass through their mother’s birth canal.
Just a few years ago on February 15th, 2001 we saw our first glimpse of a complete human genome published in the journal Nature. What we thought was a blueprint for solving the mysteries of human disease quickly clouded our concept of the things that us grant us a long and healthy life. Numerous studies appearing in prestigious journals have shown us again and again that genetics plays only a small role in the outcome of our lives[7] [8] [9]. In fact, as little as 10% of our health and longevity is determined by our genetic programming. The rest comes from the daunting myriad of external influences encompassed by entire universes about which we know very little. After all, we’ve only cultured and identified less than 1% of the life within soil, which carries the same microorganisms as our bodies. The rest is still a mystery. Perhaps understanding them from a scientific basis carries little merit. Wisdom has been passed down in many forms. The original innate wisdom requires no explanation. Its knowledge lies in the very fact that we share this space and time with millions of other creatures whose DNA also survived this epic journey. Because they are here, we are here. Compliments of the fierce compassion of our mothers who carried forth pieces of The Living Goddess.
[1] Darwin, Charles M.A. On the Origin of Species by Means of Natural Selection, or the Preservation of Favoured Races in the Struggle for Life. 24 November 1859. Nature 1st ed.) (London: John Murray) p. 503
[2] Lavialle C, Cornelis G, Dupressoir A, Esnault C, Heidmann O, Vernochet C, Heidmann T. (Aug 2013). “Paleovirology of ‘syncytins’, retroviral env genes exapted for a role in placentation.”. Philos Trans R Soc Lond B Biol Sci. 368 (1626). doi:10.1098/rstb.2012.0507. PMID 23938756
[3] Esnault C, Cornelis G, Heidmann O, Heidmann T (2013) Differential Evolutionary Fate of an Ancestral Primate Endogenous Retrovirus Envelope Gene, the EnvV Syncytin, Captured for a Function in Placentation. March 23, 1013. PLoS Genet 9(3): e1003400. doi:10.1371/journal.pgen.1003400
[4] Lederberg J, McCray AT. ’Ome Sweet ’Omics—a genealogical treasury of words. Scientist. 2001;15:8
[5] The NIH HMP Working Group. 2009. The NIH Human Microbiome Project. Genome Res. 2009 December; 19(12): 2317–2323.
[6] University of Georgia “Healthy Intestinal Bacteria Found Within Chicken Eggs.” Science Daily June 3 2008.
[7] Prof Salim Yusuf DPhil,Steven Hawken MSc,Stephanie Ôunpuu PhD,Tony Dans MD,Alvaro Avezum MD,Fernando Lanas MD,Matthew McQueen FRCP,Andrzej Budaj MD,Prem Pais MD,John Varigos BSc,Liu Lisheng MD,on behalf of the INTERHEART Study Investigators Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case-control study
The Lancet – 11 September 2004 ( Vol. 364, Issue 9438, Pages 937-952 )
DOI: 10.1016/S0140-6736(04)17018-9
[8] Mozaffarian D, Kamineni A, Carnethon M, Djoussé L, Mukamal KJ, Siscovick D. Lifestyle Risk Factors and New-Onset Diabetes Mellitus in Older Adults: The Cardiovascular Health Study. Arch Intern Med. 2009;169(8):798-807. doi:10.1001/archinternmed.2009.21.
[9] Steingraber, Sandra. Living Downstream. Reading, Mass., Addison–Wesley, 1997 “80% of all cancer is attributable to environmental [external] influences.”(Steingraber, 1998 p 60)
Phenotype (fē-nō-tīp)
noun
The net result of the interaction of an organism’s genes with its environment.
In 1953, Watson and Crick’s discovery of DNA was a beacon of hope for understanding what causes human disease. Since then science and medicine have invested billions in research and man hours under the premise and promise that understanding our genetic code would lead us to answers and cures for the leading causes of disease and death. To our surprise, the results have not been so straightforward. As we’ve gained more and more information about our genetic programming, we’ve discovered that genetics plays only a small role in the development of many of the leading causes of chronic disease and premature death. Our antiquated belief that we are destined to fall victim to a disease that ended the life of our parents and/or grandparents has given way to the sometimes difficult realization that we have more influence over the future of our health and our lifespan than we could have imagined.
As more and more research has come online, we’ve discovered that many human diseases are largely a result of external factors that are potentially under our control. A study published in 2004 in The Lancet followed over 15,000 people assessing risk factors for heart attack. The authors identified nine non-genetic risk factors that “collectively accounted for 90-94% of cardiovascular disease and had the potential to prevent the majority of premature myocardial infarction1”. These risk factors were composed of external influences that can all be eliminated including “Abnormal lipids, smoking, hypertension, diabetes, abdominal obesity, psychosocial factors, consumption of fruits, vegetables, and alcohol, and regular physical activity[i]”.
A study appearing in JAMA in 2008 on 4883 people over the age of sixty-five concluded that 90% of DM2 cases are preventable using 5 lifestyle changes. Diabetes-related risk factors include physical activity level, dietary habits, adiposity, alcohol use, and smoking habits[ii].
As our understanding deepens, it is becoming apparent that perhaps these are not diseases at all but in fact what we call phenotypes.
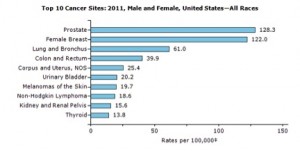
Since 1998, the statistics regarding cancer risk, which were studied separately by the NIH and WHO, have remained surprisingly steady. Despite thousands of new studies every year the figures stood at approximately 80% environmental (a scientific term for external factors) and 20% genetic. This was concurred in 2014 by The American Cancer Society saying, “environmental factors (as opposed to heredity factors) account for an estimated 75%-80% of cancer cases and deaths in the US[iii]. On January 2nd 2015 this assessment came crashing down with the controversial Science article by Cristian Tomasette and Bert Vogelstein titled “Variation in Cancer Risk Among Tissues Can Be Explained by the Number of Stem Cell Division”[iv]. This was an elegant, groundbreaking study that shined a light on the novel idea that some cancers simply occur because of random mutations during stem cell division. Suddenly, part of the 80% environmental aspect had to be redefined. The authors’ unfortunate choice to assign a new value to the environmental influence in the absence of adequate data parameters was incendiary across the media and scientific community. Six of the top eleven most frequently occurring cancer types were not included in this study. Interestingly, each of the excluded cancer types have a huge body of scientific evidence demonstrating that each of them is highly influenced by environmental factors. Among these cancers were prostate, breast, uterine, urinary bladder, kidney and Non Hodgkin’s Lymphoma, collectively, responsible for nearly 20% or 1/5 of cancer deaths in the US in 2014 and their incidence rate an even higher contribution3. The environmental factors that influence their development include infectious agents[v], endogenous[vi] and exogenous hormones, xenobiotic compounds[vii], certain heavy metals[viii], certain pharmaceuticals, specific industrial and organic chemicals[ix], alcohol consumption[x], glycemic control[xi], and aflatoxin[xii].
One reason the scientific community raised such a fuss about the “bad luck” cancer study was that an inordinate amount of funding and resources is already dedicated to the diagnosis and treatment of cancer. The same goes for many other “diseases” including heart disease and diabetes. After all, each one forms a massive economic base that generates billions of dollars annually. Research funding directed towards the understanding and true prevention of these diseases contributes very little to monthly recurring revenues. Instead, it represents an ominous threat to the economic base of the medical industry as well as any industry whose products might be identified as a risk. Despite the hurdles, advances in our understanding of the processes that create these “diseases” has accelerated so fast that it has created a growing chasm where science and medicine no longer overlap but have diverged. The statistics about the environmental influences on “disease” have been well known in the scientific community for at least 15 years. However, they are poorly acknowledged by the medical industry and, as a result, have remained clandestine to the general public. Chemicals aside, imagine if society truly understood how they could prevent diabetes or delay the onset of heart disease simply by adopting a regimen of glycemic control as described in the studies above. What if it was not based on a drug but was based on reducing their consumption of excess sugar? This one change would have massive reverberations through multiple industries. On one side, there would be reduced “need” for medical services that manage the entire sequela of diseases that are known to be caused by poor glycemic control. This would translate into reduced doctor visits, reduced “need” for pharmaceuticals, fewer hospitalizations, fewer surgeries, lower consumption medical supplies, reduced need for assisted living and in home care, reduction of insurance costs etc. On the other side the industrial farming and food complex would also be widely affected. This includes farming equipment, GPS equipment, chemical fertilizers, pesticides, herbicides, fungicides, GMO seeds, all sugar-laden products, packaging, transportation and distribution, fuel consumption etc. As you can see, a significant base of the economy relies on a mutualistic relationship between Big Farma and Big Pharma. The current medical paradigm actually benefits from environmental problems and generally relegates efforts to fix this to the realm of environmental fundamentalism and quackery.
At what point do we embrace our responsibility of removing the known causes of disease? There are already billions of dollars and man-hours wasted on researching and treating diseases that are created by humans literally poisoning themselves. What is the sense? To continue to protect economic interests cloaked inside a societal dietary lexicon that has been hijacked by mass manipulation of naturally occurring, animalistic addictions through marketing, food additives and advertising? We must focus on removing the factors that create these disease phenotypes. Once this illusion has been cleared we can direct our resources towards novel drugs and therapies that will do the most good. Image a healthy, thriving society where disabled life expectancy is a thing of the past. Where companies and organizations like SENS, Calico and Human Longevity Inc. create drugs that don’t depend on illness but address the factors that are not under our control to produce meaningful lasting advances in health and longevity.
[i] Prof Salim Yusuf DPhil,Steven Hawken MSc,Stephanie Ôunpuu PhD,Tony Dans MD,Alvaro Avezum MD,Fernando Lanas MD,Matthew McQueen FRCP,Andrzej Budaj MD,Prem Pais MD,John Varigos BSc,Liu Lisheng MD,on behalf of the INTERHEART Study Investigators Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case-control study
The Lancet – 11 September 2004 ( Vol. 364, Issue 9438, Pages 937-952) DOI: 10.1016/S0140-6736(04)17018-9
[ii] Dariush Mozaffarian, MD, DrPH; Aruna Kamineni, MPH; Mercedes Carnethon, PhD; Luc Djoussé, MD, ScD; Kenneth J. Mukamal, MD; David Siscovick, MD, MPH. Lifestyle Risk Factors and new Onset Diabetes Mellitus in Older Adults. Arch Intern Med. 2009;169(8):798-807. doi:10.1001/archinternmed.2009.21.
[iii] [iii] ACS (2014). Cancer Facts & Figures 2014, Atlanta. American Cancer Society, 2014. Available at: http://www.cancer.org/acs/groups/content/@research/documents/webcontent/acspc-042151.pdf
[iv] Cristian Tomasetti, Bert Vogelstein. Variation in Cancer Risk Among Tissues Can Be Explained by the Number of Stem Cell Divisions. Science 2 January 2015 Vol. 347 no. 6217 pp. 78-81. DOI:10.1126/science.1260825
[v] Yidya Vedham Ph. D., Mukesh Verma Ph. D. Cancer-Assoicated Infectious Agents and Epigenetic Regulation Cancer Epigenetics Methods in Molecular Biology Nov. 8, 2014 Vol. 1238, pp333-354 Doi: 10.1007/978-1-4939-1804-1_18
[vi] Tim Key; Endogenous Hormones Breast Cancer Collaborative Group Steroid hormone measurements from different types of assays in relation to body mass index and breast cancer risk in postmenopausal women: Reanalysis of eighteen prospective studies. Steroids. Oct. 7, 2014. Doi: 10.1016/j.steroids.2014.09.001
[vii] Hye-Rim Lee; Kyung-A Hwang; Kyung-Chul Choi. The estrogen receptor signaling pathway activated by phthalates is linked with transforming growth factor-β in the progression of LNCaP prostate cancer models. International Journal of Oncology. May 22, 2014. Pp595-602 Doi: 10.3892/ijo.2014.2460
[viii] García-Lestón, J; Roma-Torres, J; Vilares, M; Pinto, R; Prista, J; Teixeira, JP; Mayan, O; Conde, J; Pingarilho, M; Gaspar, JF; Pásaro, E; Méndez, J; Laffon, B. Genotoxic effects of occupational exposure to lead and influence of polymorphisms in genes involved in lead toxicokinetics and in DNA repair. Environ Int, 2012 vol. 43 pp. 29-36
[ix] Guo, H; Bassig, BA; Lan, Q; Zhu, Y; Zhang, Y; Holford, TR; Leaderer, B; Boyle, P; Qin, Q; Zhu, C; Li, N; Rothman, N; Zheng, T. Polymorphisms in DNA repair genes, hair dye use, and the risk of non-Hodgkin lymphoma. Cancer Causes Control, 2014 vol. 25(10) pp. 1261-70
[x] Qian Zhong, Ganggang Shi, Yanmei Zhang, Lei lu, Daniel Levy, Shuping Zhong. Alteration of BRCA1 Expression Affects Alcohol-induced Transcription of RNA Pol III-Dependent Genes. Gene Vol 556, Issue 1, Feb. 1, 2015 74-79.
[xi] Juhyun Park; Sung Yong Cho; Young Ju Lee; Seung Bae Lee; Hwancheol Son; Hyeon Jeong. Poor Glycemic Control of Diabetes Mellitus Is Associated with Higher Risk of Prostate Cancer Detection in a Biopsy Population. PLOS Sept. 18, 2014. Doi: 10.1371/journal.pone.0104789
[xii] Xi-Dai Long; Dong Zhao; Xiao-Qiang Mo; Chao Wang; Xiao-Ying Huang; Jin-Guang Yao; Yun Ma; Zhong-Hua Wei; Min Liu; Li-Xiao Zeng; Jian-Jun Zhang; Feng Xue; Bo Zhai; Qiang Xia. Genetic Polymorphisms in DNA Repair Genes XRCC4 and XRCC5 and Aflatoxin B1–related Hepatocellular Carcinoma. Epidemiology Sept 2013, Vol. 24 Issue 5 pp. 671-81. Doi: 10.1097/EDE.0b013e31829d2744
In follow up to my previous post outlining concerns and conflicts to the recent “Bad Luck” cancer study, here is a technical article that I submitted to the journal Science. For whatever reasons, they did not want to publish it. Some of the original misinterpretations were brought about by the news editor’s summary. Here is my original manuscript complete with references for the conflicts between conclusions and the study parameters.
This is a game changing study and its wording is of delicate importance. Previous to this study, the NIH and CDC had concluded that 85-90% of cancer cause is due to external factors with only a small percentage being attributed to genetics. Despite this, an inordinate amount of funding has been dedicated to detection and treatment instead of prevention. This study has the power to influence how much energy, funding and resources are dedicated to understanding and eliminating the environmental (external) causes of cancer.
Acknowledgements:
Deepest gratitude to Rebe Feraldi MS, LCACP and Jessee Carrato for their valuable insights and also to Rebe for her time to do a thorough review.
Rebe and I subsequently submitted a letter to Science asking for clarification.
____________________
Abstract:
The recent study, Variation in Cancer Risk Among Tissues Can Be Explained by the Number of Stem Cell Divisions. , published in the January 2, 2015 edition of Science by Cristian Tomasette and Bert Vogelstein elegantly shines light on the previously overlooked notion that many cancer types arise randomly from errors during stem cell divisions.
However, as outlined below, there is a significant dissonance between the study’s data parameters and the statements and conclusions set forth in the study’s title and abstract. As a member of (American Association for the Advancement of Science) AAAS, I feel it is in the spirit of science to demand clear, objective articles whose data and conclusions are congruous and are not overtly vulnerable to misinterpretation by the vast majority of the media, science writers, and the public. Of further concern, these statements could be deceiving for those making informed decisions regarding research funding and public policy.
The primary aspect of the study data parameters which lacks congruity with the study conclusions and abstract is as follows: Six of the top eleven most frequently occurring cancer types were not included in this study and are thus, not reflected in the study conclusions. Among these cancers are: prostate, breast, uterine, urinary bladder, kidney and Non Hodgkin’s Lymphoma, collectively, responsible for nearly 20% or 1/5 of cancer deaths in the US in 2014 and their incidence rate an even higher contribution.[1] There is a substantial body of evidence demonstrating that each of these omitted cancer types is highly influenced by a variety of environmental factors. These environmental factors include infectious agents[2], endogenous[3] and exogenous hormones, xenobiotic compounds[4], certain heavy metals[5], certain pharmaceuticals, specific industrial and organic chemicals[6], alcohol consumption[7], glycemic control[8], and aflatoxin[9]. Per the American Cancer Society (ACS), “environmental factors (as opposed to heredity factors) account for an estimated 75%-80% of cancer cases and deaths in the US.”[10] Although we may eventually find that these environmental factors drive accelerated “random” error events, it is not possible to draw an objective, statistically meaningful conclusion about environmental and heredity vs. “random” influences on cancer as a whole based on the limited data parameters set forth in this study.
Although the aforementioned cancers types (excluded from the study) are presumably part of the one-third of “tissues [that] were not included in [the] analysis”12, their predominance represents a substantial and statistically significant percentage of cancers in the US. It is understandable that “the requisite parameters were not found in the literature or [their] estimation was difficult to derive”12. However, in light of these major exclusions, the conclusions drawn by the authors in the title and in the abstract of the paper should reflect this gross exclusion. Because the exclusion is not highlighted, the stated study conclusions are ambiguous and, as we have seen in the media, are vulnerable to misinterpretation.
Furthermore, the random cancer incidents demonstrated in the study do not parallel the incidence of various cancer types reported by the CDC and NIH. In some cases, like lung and colorectal cancer, they are flip-flopped. This suggests that influences exist outside of the random events like immunity[11] and well-known tumor suppressor genes, which are not accounted for in the study. The article should clarify that the study conclusion merely represents a statistical probability of a random cancer cell events in a given tissue type over a lifetime but does not represent the ultimate fate of any cancer type or cell. The data presented from the study does not provide sufficient evidence for the following statement in the abstract:
“These results suggest that only a third of the variation in cancer risk among tissues is attributable to environmental factors or inherited predispositions. The majority is due to “bad luck,” that is, random mutations arising during DNA replication in normal, noncancerous stem cells. This is important not only for understanding the disease but also for designing strategies to limit the mortality it causes.[12]”
Did the authors intend that their conclusion was based only on “tissues” that were included in their study? Or were they implying that “only one third of [all] cancer risk is attributable to environmental factors or inherited predispositions?12” Below is a sampling from hundreds of various headlines from six prestigious media organizations that understandably misinterpreted the conclusion of the study article.
******
“Most cancers are ’caused by bad luck – not lifestyle’: Scientists claim 65% of cases are down to random mistakes in genes that we can do nothing about” Jenny Hope of the Daily Mail Jan. 1 2015
“Two-Thirds of Cancer Due to Bad Luck, Study Finds” by Mary Elizabeth Dallas of CBS News reported Jan. 1 2015
“Most cancers are caused by bad luck not genes or lifestyle, scientists say.” By Sara Knapton for the Telegraph Jan. 1 2015
“Bad luck of random mutations plays predominant role in cancer”. Science Daily Jan. 1 2015
“Scientists: Random Gene Mutations Primary Cause of Most Cancer” by Ben Brumfield on CNN
“Biological bad luck blamed in two-thirds of cancers” by Will Dunham of Reuters
******
In addition to the ambiguity of the abstract, the title implies the study is based on overall cancer risk. It would be more accurate to change the wording from “cancer risk” to “some cancer types”.
Of further concern, the authors also make recommendations that could influence the direction and funding of research and public policy. Because of the excluded types of cancer and overall data, the following statement from the article is not scientifically objective and should be removed or clarified:
“Moreover, we show that these stochastic influences are in fact the major contributors to cancer overall, often more important than either hereditary or external environmental factors.”
The authors go on to conclude that,
“These results suggest that only a third of the variation in cancer risk among tissues is attributable to environmental factors or inherited predispositions.”
Although compelling, it is premature to assign exact figures and conclusions based on such limited data.
Ultimately, because of its limited data parameters, this study lacks the merit necessary for forming decisions around research funding and public policy. The broad statements in this article could be misinterpreted by non-science policy makers potentially leading to uninformed decisions about a wide range of issues from allocation of research funds to public health recommendations.
Because the media headlines were so grossly misinterpreted and misleading to the public, corrections and clarifications should be made available as a press release. This act would be in the interest of the integrity of the scientific community and in good faith to the non-science public.
[1] Based on calculations from statistical data in ACS (2014).
[2] Yidya Vedham Ph. D., Mukesh Verma Ph. D. Cancer-Assoicated Infectious Agents and Epigenetic Regulation Cancer Epigenetics Methods in Molecular Biology Nov. 8, 2014 Vol. 1238, pp333-354 Doi: 10.1007/978-1-4939-1804-1_18
[3] Tim Key; Endogenous Hormones Breast Cancer Collaborative Group Steroid hormone measurements from different types of assays in relation to body mass index and breast cancer risk in postmenopausal women: Reanalysis of eighteen prospective studies. Steroids. Oct. 7, 2014. Doi: 10.1016/j.steroids.2014.09.001
[4] Hye-Rim Lee; Kyung-A Hwang; Kyung-Chul Choi. The estrogen receptor signaling pathway activated by phthalates is linked with transforming growth factor-β in the progression of LNCaP prostate cancer models. International Journal of Oncology. May 22, 2014. Pp595-602 Doi: 10.3892/ijo.2014.2460
[5] García-Lestón, J; Roma-Torres, J; Vilares, M; Pinto, R; Prista, J; Teixeira, JP; Mayan, O; Conde, J; Pingarilho, M; Gaspar, JF; Pásaro, E; Méndez, J; Laffon, B. Genotoxic effects of occupational exposure to lead and influence of polymorphisms in genes involved in lead toxicokinetics and in DNA repair. Environ Int, 2012 vol. 43 pp. 29-36
[6] Guo, H; Bassig, BA; Lan, Q; Zhu, Y; Zhang, Y; Holford, TR; Leaderer, B; Boyle, P; Qin, Q; Zhu, C; Li, N; Rothman, N; Zheng, T. Polymorphisms in DNA repair genes, hair dye use, and the risk of non-Hodgkin lymphoma. Cancer Causes Control, 2014 vol. 25(10) pp. 1261-70
[7] Qian Zhong, Ganggang Shi, Yanmei Zhang, Lei lu, Daniel Levy, Shuping Zhong. Alteration of BRCA1 Expression Affects Alcohol-induced Transcription of RNA Pol III-Dependent Genes. Gene Vol 556, Issue 1, Feb. 1, 2015 74-79.
[8] Juhyun Park; Sung Yong Cho; Young Ju Lee; Seung Bae Lee; Hwancheol Son; Hyeon Jeong. Poor Glycemic Control of Diabetes Mellitus Is Associated with Higher Risk of Prostate Cancer Detection in a Biopsy Population. PLOS Sept. 18, 2014. Doi: 10.1371/journal.pone.0104789
[9] Xi-Dai Long; Dong Zhao; Xiao-Qiang Mo; Chao Wang; Xiao-Ying Huang; Jin-Guang Yao; Yun Ma; Zhong-Hua Wei; Min Liu; Li-Xiao Zeng; Jian-Jun Zhang; Feng Xue; Bo Zhai; Qiang Xia. Genetic Polymorphisms in DNA Repair Genes XRCC4 and XRCC5 and Aflatoxin B1–related Hepatocellular Carcinoma. Epidemiology Sept 2013, Vol. 24 Issue 5 pp. 671-81. Doi: 10.1097/EDE.0b013e31829d2744
[10] ACS (2014). Cancer Facts & Figures 2014, Atlanta. American Cancer Society, 2014. Available at: http://www.cancer.org/acs/groups/content/@research/documents/webcontent/acspc-042151.pdf
[11] Junko Kishikawa, Kazushige Kawai, Nelson Tsuno, Hironori Yamaguchi, Soichiro Ishihara, Eiji Sunami, Toshiaki Watanabe. Characteristics and Pronosis of Colorectal Cancer Assoicated with Rheumatic Disease. International Surgery, Dec. 30, 2014 , Doi: 10.9738/INTSURG-D-14-00154.1
[12] Cristian Tomasetti, Bert Vogelstein. Variation in Cancer Risk Among Tissues Can Be Explained by the Number of Stem Cell Divisions. Science 2 January 2015 Vol. 347 no. 6217 pp. 78-81. DOI:10.1126/science.1260825
Acknowledgements:
Deepest gratitude to Rebe Feraldi MS, LCACP and Jessee Carrato for their valuable insights and also to Rebe for her time to do a thorough review.
A new study on the “bad luck” of cancer is a wonderful contribution to science but is being severely misinterpreted by both science writers and the media.
This study[1] is the first of its kind to accurately quantify the probability of the development of a cancer cell in any given tissue over a lifetime. It supports other hypotheses[2] stating that increased frequency of cell division, which is also a hallmark of cellular aging, leads to increased risk of cancer. However, it is not representative of a cancer cell’s ultimate destiny. New cancer cells form in our bodies everyday and our immune system destroys them. We’ve known for 20+ years that tissues that are prone to faster cell division and turnover, like colon and skin, have a higher probability of developing cancer cells. This is why inflammation is strongly associated with the development of cancer. Inflammation from immune activity causes rapid damage and therefore places a high demand on the affected tissue for renewal by cell division. Thus, a higher frequency cell division results in a higher statistical probability that mutations will occur, cancer cells will develop and one of them might escape under the immune system’s radar. An example; we know that cigarette smoking leads to a much higher risk of developing lung cancer. There are two parts to this. One is the simple carcinogenicity of some of the chemicals in cigarette smoke. However, a much larger role is played by the fact that the body responds to cigarette smoke by launching an immune response that leads to increased inflammation, increased cellular replacement, impaired cellular death and diminished tissue cleanup[3],[4].
Most importantly, the cancer risk study excludes any statistics on breast and prostate cancers. Perhaps these were intentionally excluded from the study as edge cases since their occurrence in the general population is 300% higher than any of the cancers included in the study. Because these tissues are hormone-sensitive, they are highly susceptible to influence by external factors. Hundreds of studies have established their extreme vulnerability to chemicals that mimic estrogen and stimulate rapid growth and cell division. This parallels the theme of this recent study suggesting that faster cell division leads to a high probability of mutations and cancer cell development. Including these two types of cancer in the study’s statistics could increase their external influence factor by as much as 20%.
Although there are some correlations it is important to note that the probabilities in the study do not evenly parallel rates of cancer incidence in the US. For example the study shows, stem cell divisions in colorectal cells as being significantly higher but than in lung but cancer statistics show that the incidence of these cancers is flip flopped.
While this information is invaluable for quantifying cancer cell development, it is missing significant aspects of our basis of cancer knowledge and statistics and by no means establishes final numbers or parameters for cancer risk or its influence by external factors.
[1] Cristian Tomasetti, Bert Vogelstein. Variation in Cancer Risk Among Tissues can be Exlapined by the Number of Stem Cell Divisions. Science 2 January 2015 Vol. 347 no. 6217 pp. 78-81. DOI: 10.1126/science.1260825
[2] Steve Horvath. DNA Methylation Age of Human Tissue and Cell Types. Genome Biology 2013 12:R115 doi:10.1186/gb-2013-14-10-r115
[3] Naotaka Noda, Koichiro Matsumoto, Soturu Fukuyama, Yukari Asia, Hiroko Kitajima, Nanae Seki, Yuko Matsunaga, Keiko Kan-o, Atsushi Moriwaki, Konosuke Morimoto, Hiromasa Inoue and Yoichi Nakanishi. Cigarette Smoke Impairs Phagocytosis of Apoptotic Neutrophils by Alveolar Macrophages Via Inhibition of Histone Deacetylase/Rac/CD9 Pathways. Int. Immunol. (2013) 25 (11) 643-650. doi: 10.1093/intimm/dxt033
[4] Susan JM Hoonhorst, Wim Timens, Leo Koenderman, Adele T Lo Tam Loi, Jan-Willem J Lammers, H Marike Boezen, Antoon JM van Ossterhout, Dirkje S Postma, Nick HT ten Hacken. Increaded Activation of Blook Neutrophils After Cigarette Smoking in Young Individuals Susceptible to COPD. Respiratory Research 2014 15:121 doi:10.1186/s12931-014-0121-2